Bi-Monthly Injectable HIV-Treatment Drug Cabenuva Listed On Australia’s PBS
The world’s first long-acting treatment for HIV, Cabenuva has been listed on the Pharmaceutical Benefits Scheme (PBS) for Australians living with HIV.
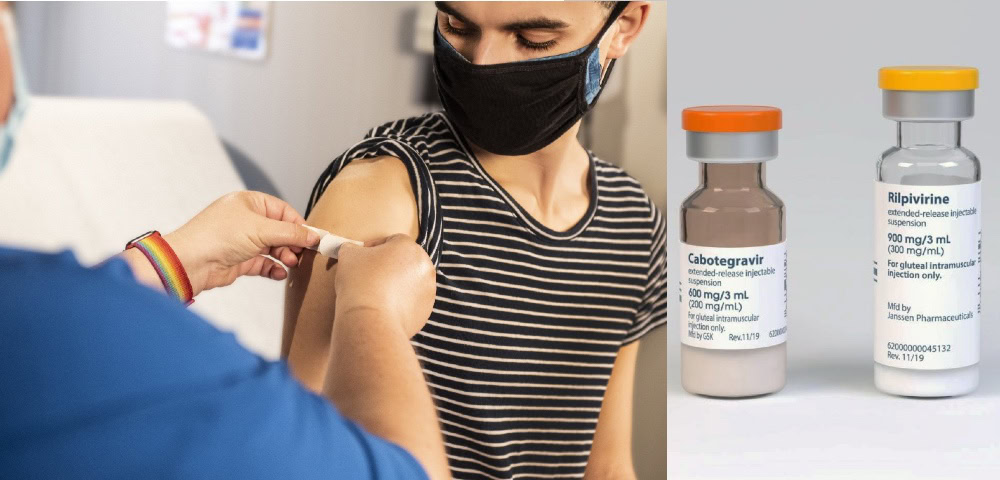
ViiV Healthcare’s Cabenuva, a combination of Cabotegravir and Rilpivirine, is an injectable drug that is administered once every two months. This is a huge change for around 29,000 Australians who are living with HIV, who currently take oral antiretroviral therapy (ART) daily.
Cabenuva is a single three mL (600 mg) IM injection of cabotegravir and a single three mL (900 mg) IM injection of rilpivirine. The treatment regiment recommended is a one-month oral lead-in with cabotegravir 30 mg and rilpivirine 25 mg tablets taken together with a meal. On the final day of the oral-lead in, Cabenuva is administered. Following an initiation injection in the third month, Cabenuva is administered every two months from the fifth month onwards.
A Long-Acting Injectable Option
“Some people living with HIV in certain populations, such as Aboriginal and/or Torres Strait Islander people, those living in rural or remote settings, and individuals with complex living or social circumstances, had issues adhering to a daily oral regimen and would potentially have improved quality of life from a long-acting injectable option,” the Pharmaceutical Benefits Advisory Committee (PBAC) said while recommending that Cabenuva be listed on the PBS.
Six doses of Cabenuva will be reimbursed every year for adults living with HIV, who have “no known or suspected resistance to either cabotegravir or rilpivirine.”
“The approval of long-lasting injectable anti-retroviral treatments are a welcome development for people with HIV,” Scott Harlum, President of the National Association for People with HIV in Australia (NAPWHA), said in a statement.
“As an additional treatment option, long-lasting injectable treatments can reduce the burden of taking daily medication and further assist people with HIV adhere to their treatment regime. This, in turn, helps ensure people with HIV are able to maintain an undetectable viral load which is both good for their own health but also eliminates any risk of transmission of HIV,” said Harlum.
“Long acting injectable HIV treatment is a great advancement that helps free up the lives of people living with HIV, and adding it to the PBS makes it a more accessible option for Australians,” said Simon Ruth, CEO. Thorne Harbour Health.
A Safe And Convenient Option
“Cabenuva is an injectable regimen and that is significant for people who can’t, or struggle to, take tablets and for people travelling overseas where carrying HIV treatments that is not always safe or convenient,” Robert Mitchel, NAPWHA Special Representative told Star Observer.
“NAPWHA support people living with HIV having access to the broadest possible range of HIV treatments possible. NAPWHA has always supported choice for the consumer. The listing of Cabenuva gives the consumer greater choice. We welcome its listing,” added Mitchel.
The United States Food and Drug Administration had approved Cabenuva in January 2021. The safety and efficacy of the injectable drug regimen were established after two randomised, open-label and controlled trials in 1,182 HIV infected adults.
Patients who participated in both trials continued to show virological suppression, with no relevant change in their baseline in CD4+ cell counts. Some of the common adverse reactions reported during the trial were injection site reactions, fever, fatigue, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness and rash.