FDA Approves Monthly Injectable HIV Drug Combo
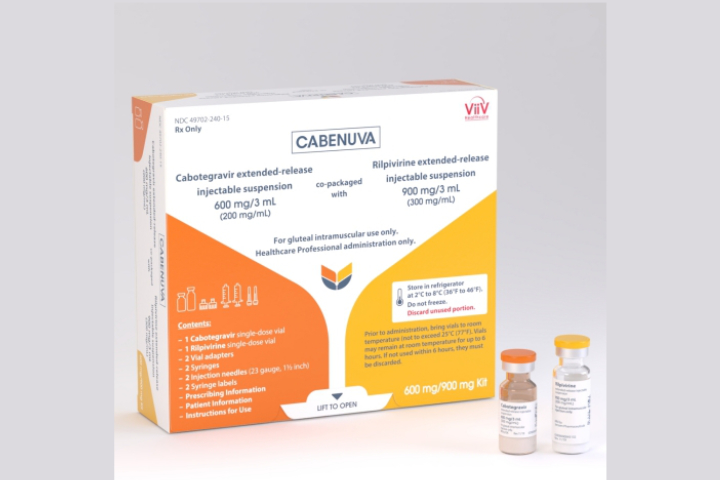
The United States Food and Drug Administration on Thursday approved Cabenuva, a once a month injectable drug combination with cabotegravir and rilpivirine, for the treatment of HIV in adults.
“Currently, the standard of care for patients with HIV includes patients taking daily pills to adequately manage their condition. This approval will allow some patients the option of receiving once-monthly injections in lieu of a daily oral treatment regimen,” said Dr. John Farley, director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research, in a statement.
Cabenuva is priced by ViiV Healthcare in the US at $5,940 for the initial higher dose and $3,960 monthly thereafter.
From 365 Days To 12 Days
Lynn Baxter, Head of North America, ViiV Healthcare that developed the medication said that it would offer a new approach to care for people living with HIV.
“Cabenuva reduces the treatment dosing days from 365 days to 12 days per year.”
PLHIV who want to switch to the month injectable drug regimen should first be put on Vocabria (cabotegravir in a tablet), which was also approved by FDA, and oral rilpivirine (Edurant), one month before starting on Cabenuva.
If the medications are well-tolerated, then they can be put on Cabenuva, that would consist of two individual intramuscular injections in the buttocks.
Safety Trials In Virologically Suppressed
FDA had done a fast track and priority review of Cabenuva and Vocabria. This followed after the safety and efficacy of the injectable drug regimen was established after two randomized, open label and controlled trials in 1,182 HIV infected adults. Those who were selected for the trials were virologically suppressed, which means a HIV-1 RNA of less than 50 copies per millilitre, before they were put on Cabenuva.
In both the trials the patients continued to show virologic suppression and there was no relevant change in their baseline in CD4+ cell counts. The common adverse reactions were injection site reactions, fever, fatigue, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness and rash.
The news about the FDA approval was welcomed across the world.
“This is a watershed moment for HIV treatment… I think about our treatment options in the 80s and 90s – high pill burden and many side effects. We’ve come a long way,” Atlanta-based HIV physician David Malebranche posted on Twitter.
This is a watershed moment for #HIV treatment.
FDA approval of an injectable, once-a-month treatment for people living with HIV.
I think about our treatment options in the 80s and 90s – high pill burden and many side effects.
We've come a long way. https://t.co/ERUap3rTnD
— David Malebranche, MD, MPH (@DMalebranche) January 21, 2021